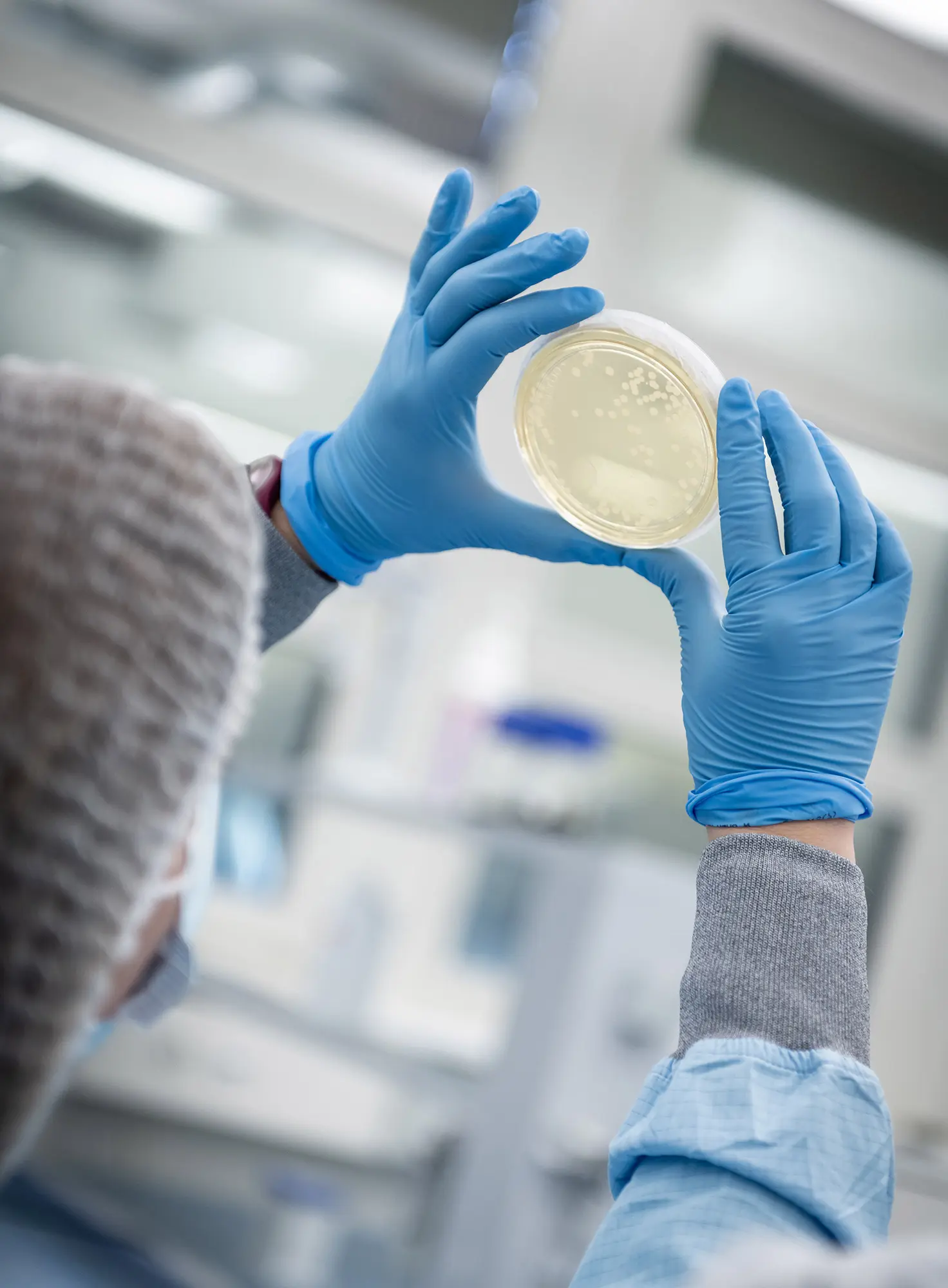
Regulatory requirements for the manufacture of pharmaceutical products can be found in many national guidelines and legal texts (E-GMP, Ph.Eur ISO, FDA) whose implementation is an integral part of ensuring a hygienic production environment. SKAN offers expert:
- Microbiological monitoring of air and surfaces according to GMP requirements from GMP Annex1, Ph Eur, ISO FDA
Supply of culture medium - Determination of total germ count structured for bacteria and yeast/moulds
- Precise identification of germs and fungi on request
- GMP-compliant documentation and analysis by a certified laboratory
- Consulting based on decades of experience with unfulfilled acceptance criteria