“Bring your innovation to its destination”
This is the guiding principle that Solvias follows every day.
At Solvias, collaboration and innovation go hand in hand, and we had the pleasure of sitting down with their team for an insightful interview. This discussion highlighted the strong partnership between Solvias and SKAN, a collaboration built on trust, expertise, and mutual dedication to advancing safety and precision in pharmaceutical and biotech processes.
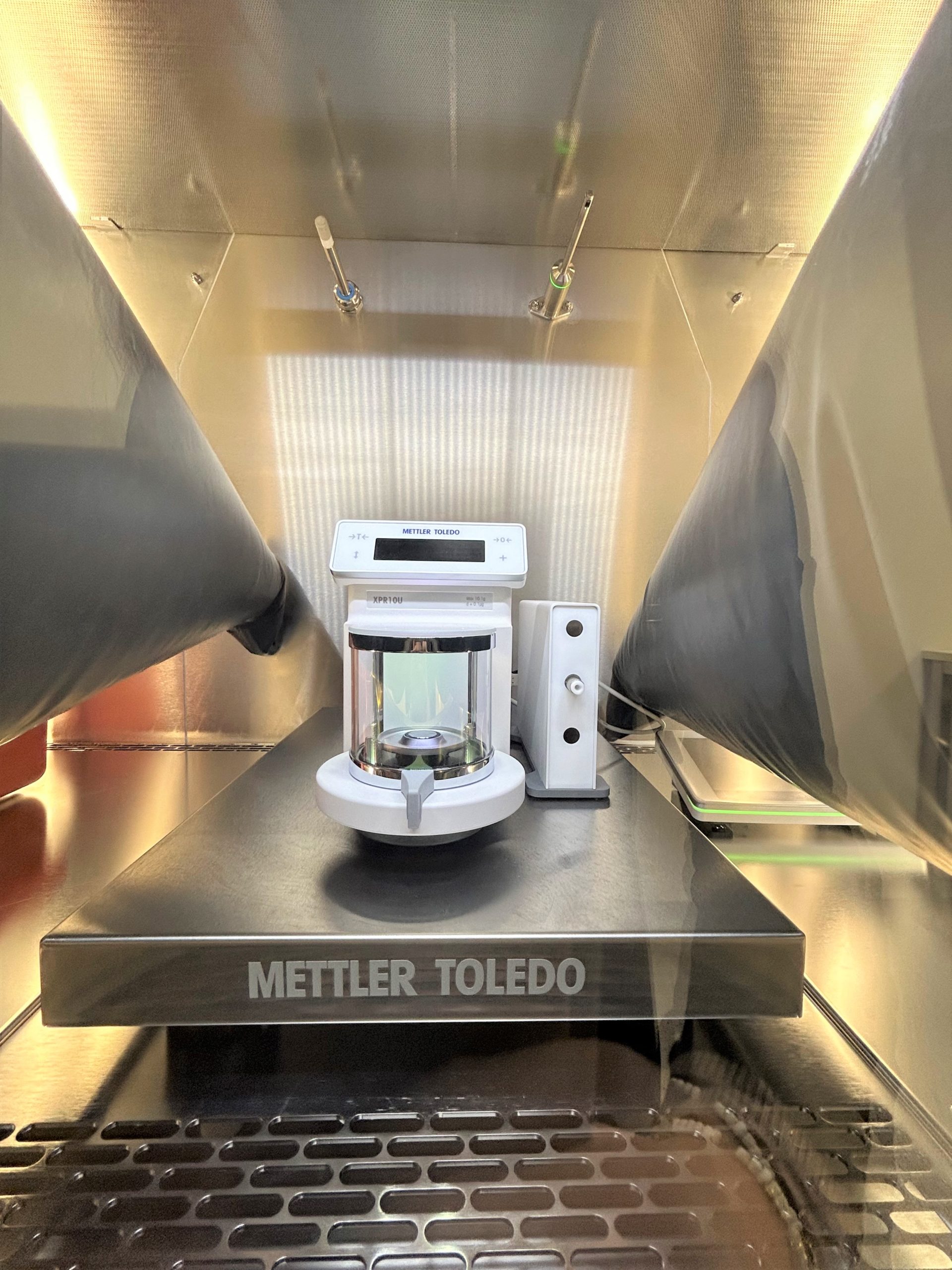
In particular, Solvias shared their experience working on the TOX 4 laboratory project, a state-of-the-art facility designed to handle highly toxic substances safely. The centerpiece of this effort is the pure2 isolator, which has exceeded expectations with its safety features, operational reliability and advanced capabilities like the integration of an ultra-microbalance from Mettler Toledo.
The interview sheds light on the seamless collaboration that made this project a success, as well as the significant steps Solvias is taking to ensure occupational safety while pushing the boundaries of analytical excellence. Read on for insights into this exciting partnership and the solutions that continue to drive innovation at Solvias.
A special thanks to Detlef Gestmann, Philip Schwarz and Charlie Brault for taking the time to share their valuable insights and experiences with us. Your feedback and collaboration are greatly appreciated!
Interview Questions
- First of all, could you describe the company Solvias in your words?
- What is the speciality/focus about the site in Kaiseraugst?
- What made you decide to partner with SKAN in this project?
- What is the scope of your department?
- How did your process look like before purchasing the pure2?
- What made you decide to move your process into an isolator?
- How does the process look like in the pure2?
- What made you decide, on the pure2 isolator?
- What has been your experience so far with the pure2?